abbott point of care covid test
Abbott Diagnostics Scarborough Inc. A positive test result for COVID-19 indicates that RNA.
Diagnostics Free Full Text Point Of Care Diagnostics In The Age Of Covid 19 Html
Abbotts rapid tests are among the most widely-used in the US with more than 200 million of our BinaxNOW and ID NOW rapid tests used in urgent care clinics doctors.

. The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or. Our test can detect COVID-19 infection regardless of strain including the delta variant. T his test is authorized for use at the Point of Care POC ie in patient care settings operating under a CLIA Certificate of Waiver Certificate of Compliance or Certificate of Accreditation.
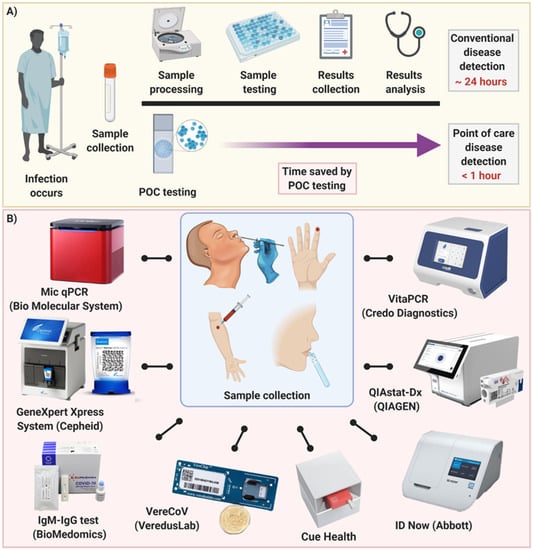
Training Resources from Test Manufacturers for COVID-19 Rapid Testing in Point-of-Care Settings Below are links to trainings developed by manufacturers of COVID-19 testing. Ensure the test is administered in qualified point-of-care setting by trained personnel The EUA for the Abbott BinaxNOWCOVID-19 Ag card test allows for use in point-of. This study recruited participants presenting for COVID-19 testing at three Melbourne metropolitan hospitals during a period of low COVID-19 prevalence.
In a single-centre laboratory evaluation study we compared AgPOCT products from seven suppliers. Food and Drug Administration FDA for the fastest available molecular point-of-care test for the. Term care residents for COVID-19.
Abbott has received emergency use authorization EUA from the US. The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or. ABBOTT Panbio Nasal Quick Reference Guide.
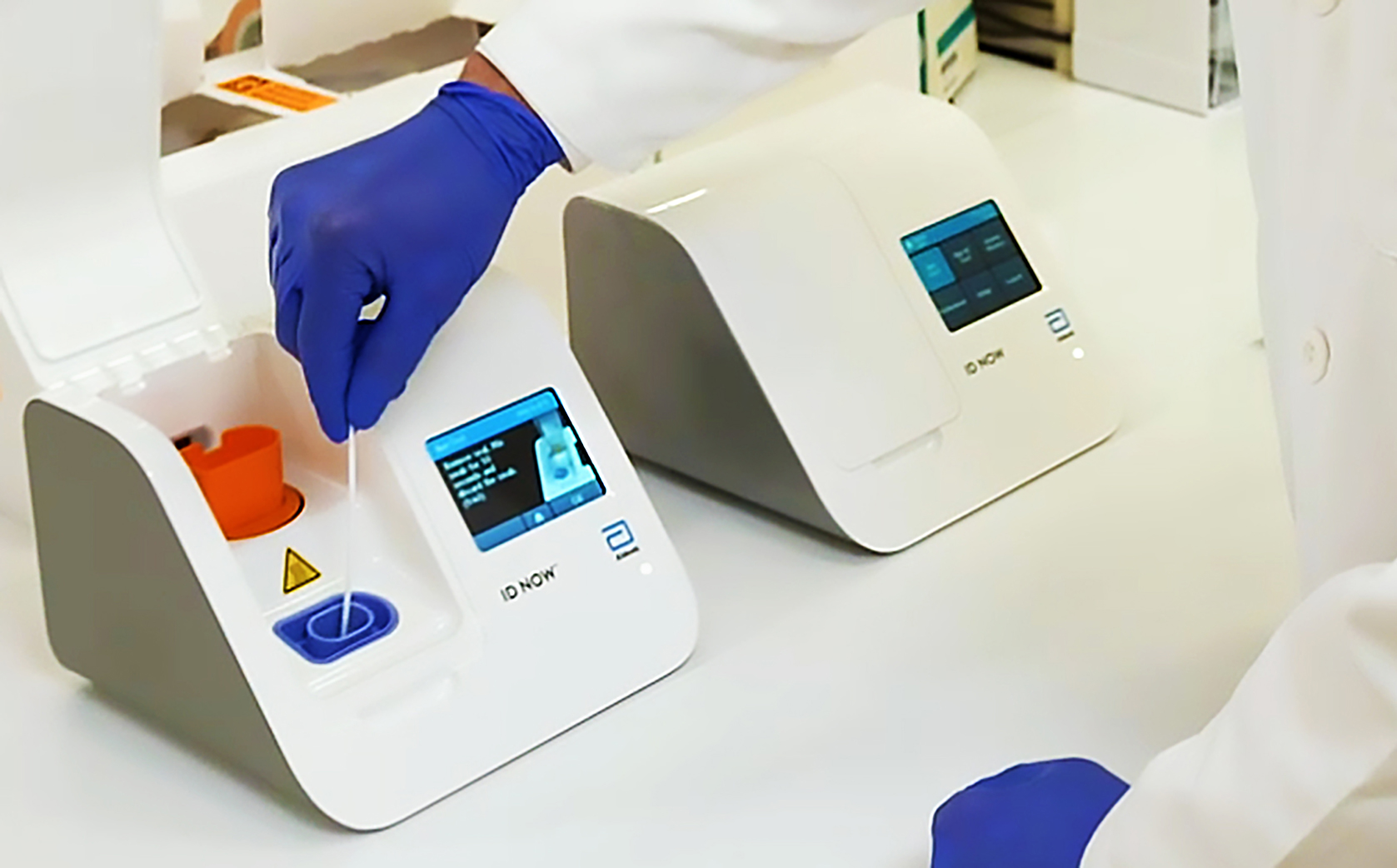
Food and Drug Administration FDA under Emergency Use. The portable rapid molecular ID NOW COVID-19 test has emerged as a critical part of this arsenal allowing fast diagnosis with results in 13 minutes or less in a variety of locations such as. Our COVID-19 unit is now making Abbott BinaxNOW COVID-19 antigen card point of care POC test kits BinaxNOW test kits available for use at nursing.
The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that. August 27 2021. The Abbott Panbio COVID-19 Ag Rapid Test the RapiGEN BIOCREDIT COVID.
Testing at the point-of-care POC for COVID-19 adds a distinct advantagerapid availability of results upon which to make treatment and infection prevention. Our test can detect COVID-19 infection regardless of strain including the delta variant. Panbio COVID-19 Ag Rapid Test Device Nasal Swab Procedure 2 minutes Read.
Point of Care POC ie in patient care settings. 4 Point of Care Testing - PanBio Nasal Process Overview. Abbott s new point-of-care test for the novel coronavirus that causes COVID-19 was approved by the US.
Ad Schedule a no-cost-to-you COVID-19 test online for select Walmart and Quest locations. The BinaxNOW COVID-19 Self Test is identical to the professional-use test used since August 2020 bringing the most studied and widely used rapid antigen test to retail shelves across the. Abbotts rapid COVID-19 test isnt the only point-of-care test to receive FDA authorization during the pandemic but Trump has touted it the most by far hailing the speed at.
Ad Schedule a no-cost-to-you COVID-19 test online for select Walmart and Quest locations. A box containing a 5-minute test for COVID-19 from Abbott Laboratories is pictured during the daily briefing on the novel coronavirus in the Rose Garden of the White House on.

14 000 Rapid Covid 19 Testing Kits Coming To Grey Bruce Ctv News

Abbott On Twitter We Re Launching A Molecular Point Of Care Test That Delivers Positive Covid 19 Results In As Little As 5 Minutes And Negative Results In 13 Minutes Providing Information Where It Is Needed
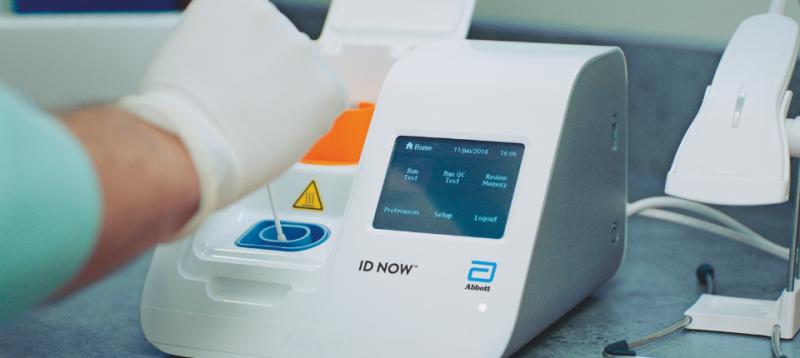
Image Gallery Showing Impact Of The Covid 19 Pandemic Daic

Rapid Testing To Expand Return Of Mass Vaccination Sites For Covid Booster Shots In B C Vernon Morning Star

Abbott Id Now 2019 Ncov Testing

Abbott Id Now Covid 19 Detection Test System Us

Point Of Care Testing Diagnostics Testing Newsroom

Demand For Abbott Labs Covid 19 Tests Soars Past 40 Million As Pandemic Cases Surge

Rapid 5 Coronavirus Test Doesn T Need Specialty Equipment Abc News
Abbott S Point Of Care Covid 19 Test Detects Coronavirus In As Little As 5 Minutes Biospace

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News

Study Raises Doubts Over Effectiveness Of Abbott Laboratories Rapid Coronavirus Test

Our Quick Guide To Rapid Covid 19 Testing Abbott Newsroom

Abbott Labs Has Shipped 566 000 Rapid Covid 19 Tests To All 50 U S States
Virus News Abbott Launches 5 Minute Covid 19 Test Bloomberg

Steps To Use Id Now Effectively Abbott Newsroom

Abbott Labs Rapid 5 Covid 19 Test To Fill In Testing Gaps For Millions In The U S

